Novem Medical: Where Expertise Meets Excellence in Medical Device Manufacturing.
Increase your medical device competitiveness by leveraging contract manufacturing and engineering services in Mexico.
Clients








Elevate precision, innovate with excellence—Novem Medical defines a new standard in medical manufacturing, where collaborative partnerships and continuous improvement pave the way for sustained success.

Cost-Effectiveness
Our strategic geographical location could yield up to 50% manufacturing costs savings when compared to in house assembly.

Access to expertise
Novem’s team leadership was forged at some of the world leading medical medical device manufacturing companies, sustaining product quality and mitigating the risks are at the core values of our team.

Flexible Production System
Flexible Production System Our extensive manufacturing capabilities paired with a high dexterity assembly team can swiftly scale up or down to support seasonal demands.

Quality Standards
Novem Medical implements rigorous quality control measures to assure regulatory compliance and while keeping the user’s safety as our priority .
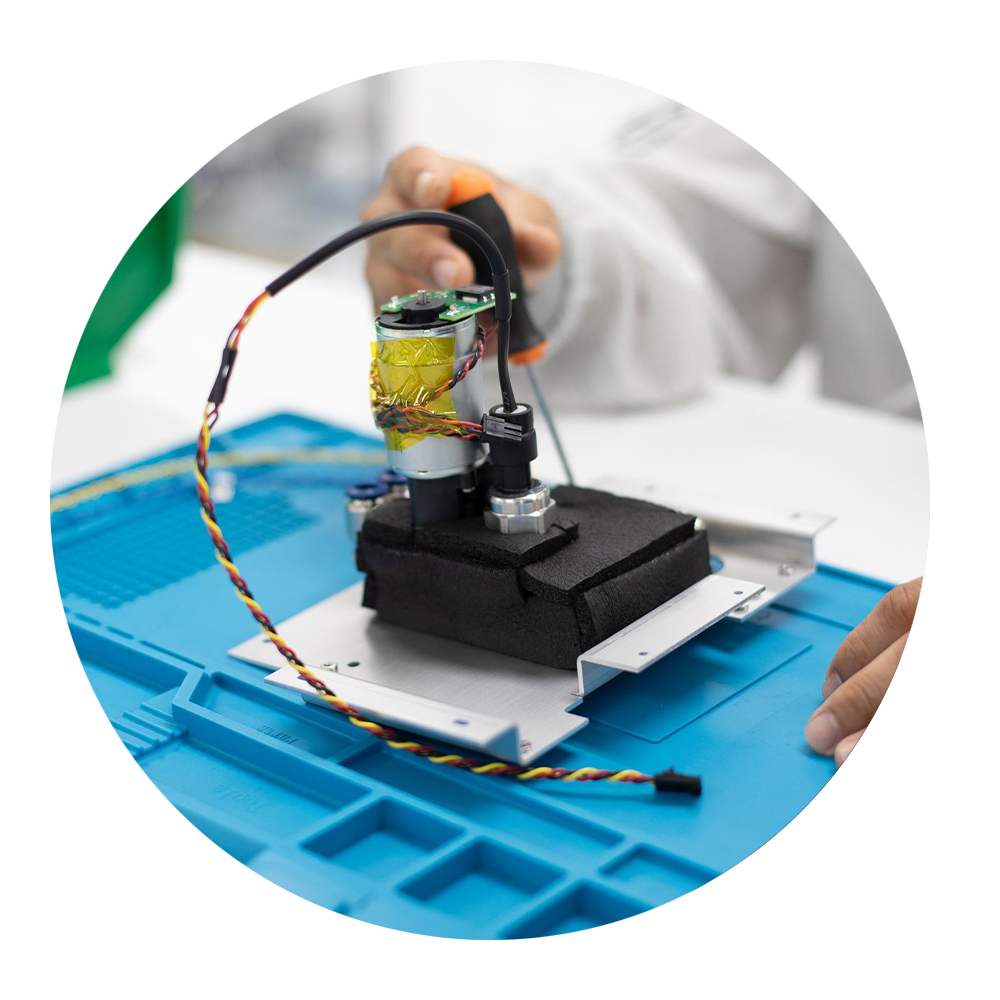
Tailored Solutions
Process optimizations often requires innovation, our NPI team specializes in working with our clients in identifying opportunities to streamline your assembly process and while maintaining the quality and compliance of your medical device.
1
Assessment and Customization
Start your medical manufacturing journey with Novem. Our experts tailor the process to meet medical industry standards collaboratively.
Contact Us
2
Innovative Manufacturing Processes
Experience top-tier medical manufacturing at Novem. Our advanced techniques, from ultrasonic welding to laser marking, guarantee precision and efficiency for your components.
3
Quality Assurance and Testing
At Novem Medical, we prioritize quality. Every manufacturing stage, including rigorous assurance like leak testing and adherence to standards such as 6 Sigma and IRCA ASQ-CQe, ensures your medical products exceed industry benchmarks.
4
Collaborative Partnership for Ongoing Excellence
A collaborative powerhouse for ongoing excellence in precision medical manufacturing. Join us in shaping the future, where passion meets precision and innovation is our hallmark.










FDA's 820 CFR (QSRs)
Novem Medical proudly adheres to the FDA's 820 CFR (Quality System Regulations), a testament to our commitment to meeting and exceeding the regulatory requirements set forth by the U.S. Food and Drug Administration.

ISO 13485:2016
Our adherence to the FDA and ISO 13485:2016 certification is a mark of distinction in the medical device industry. This certification reflects our compliance with international quality management standards, ensuring that our processes consistently meet regulatory and customer requirements.

ISO Class 8 Cleanroom
Novem Medical operates in an ISO Class 8 Cleanroom, a controlled environment designed to minimize particulate contamination during manufacturing processes. Our 500 m² cleanroom facility ensures that the air quality and cleanliness meet the stringent standards required for the production of high-precision medical components.
